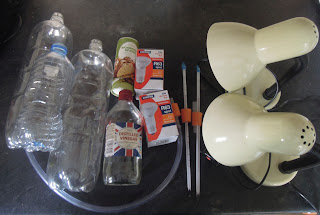
You'll need:
- 2 x 2 litre plastic bottles (these need to be identical) and 1 smaller plastic bottle.
- 2 thermometers.
- 3 corks or rubber bungs, that fit snuggly into the necks of the bottles.
- 2 lamps fitted with 100 Watt light bulbs.
- Some vingar
- Bicarbonate of soda
- A length of tubing.
- A drill
What to do:
 1. Drill a hole through the middle of each cork. Then carefully push the thermometers through two of them. They need to be a tight fit. Put one of the thermometers, mounted in a cork, into one of the 2 large plastic bottles.
1. Drill a hole through the middle of each cork. Then carefully push the thermometers through two of them. They need to be a tight fit. Put one of the thermometers, mounted in a cork, into one of the 2 large plastic bottles.2. Connect up the length of tubing to the third cork. If this is proving difficult then push the empty sheath of a ball point pen through the hole and then connect the tubing to it.

3. Put the other end of the tube into the second large bottle.
4. Now take the small bottle and spoon in 3-4 teaspoons worth of bicarbonate of soda.
5. For this step you need to be quick. Pour 200ml of vinegar on top of the bicarb, then quickly push the cork thats attached to the tubing onto the bottle. Gently swirl the vinegar/bicarb mix until it stops bubbling.
TIP: IF you can't get the cork in quickly enough, then try wrapping the bicarb up in some toilet roll. Then push the bundle into the bottle, before pouring on the vinegar.
The bicarb and vinegar react to form carbon dioxide gas. This gets pushed up and out of the tubing into the second bottle. Carbon dioxide is denser than air so it then settles in the bottom of the large bottle.
6. Now cork the large bottle with the other thermometer.
7. Take the two lamps and put them equal distance from a bottle.
8. Turn on the lamps and watch the temperature of the bottles rise.
9. The bottle containing carbon dioxide should get hotter quicker. I saw a 5 degree centigrade difference after about 10 minutes.
TIP: You need to make sure that appart from their contents the bottles are identical. Have them the same distance from the lamps and make sure the thermometers are the same depth in the bottles.
What's going on?
The gases in the bottle are transparent, that much is obvious, after all we can see through them, and we can't tell the difference between the carbon dioxide and air bottles just by looking at them. But the gasses in the bottles are only transparent to visible light. If you could see in infrared then you'd notice that the carbon dioxide bottle blocked out more of this light than the bottle containing just air. Heat can be transferred via infrared light. So as the carbon dioxide absorbs the infrared light it heats up. And the same thing is happening to our planet.




Your experiment lacks adequate controls.
ReplyDelete"So as the carbon dioxide absorbs the infrared light it heats up. And the same thing is happening to our planet."
Your conclusion goes far beyond the evidence of your experiment. What is that you are suggesting is happening to our planet?
Peter Champness