Remember those pinhole cameras I made back in June? Well they've been stuck to the side of a railway bridge since midsummer and since today is the winter solstice (in the northern hemisphere) it's time to take them down.
One camera went missing, another is was full of water. But the remaining two gave some nice images.
And here's a quick look at the results.
Has anyone else got any winter solstice solargraphs to show off?
Friday, 21 December 2012
Monday, 3 December 2012
Number 23: Dissolving eggs with washing powder
Biological washing powder contains several types of proteins called enzymes. These are naturally occurring molecules that all living things produce to do particular jobs. They are, if you like, nature’s molecular machines. There are enzymes that digest food, fight infections and replicate DNA. You name a biological process and enzymes are probably involved in it. That’s why the washing powders that contain them are ‘biological’.
The enzymes that are added to washing powders are like molecular scissors that chop up fats, starch and proteins, all of which cause stains on your clothes. The result is cleaner clothes without needing to use hot washes (because the enzymes work at low temperatures).
Its quite easy to see these enzymes in action with a nice simple demonstration using a hard boiled egg.
You'll need
You should notice that the egg in the water and the non-bio mixture hasn't changed, it still has nice sharp edges where you made the cuts. But the egg in the biological mixture is smaller and the edges are much smoother.
What's going on?
The enzymes that are added to washing powders are like molecular scissors that chop up fats, starch and proteins, all of which cause stains on your clothes. The result is cleaner clothes without needing to use hot washes (because the enzymes work at low temperatures).
Its quite easy to see these enzymes in action with a nice simple demonstration using a hard boiled egg.
You'll need
- A hard boiled egg
- 2 or 3 glasses or jars
- A knife
- A teaspoon
- Biological washing powder/liquid
- If you are in the UK you can also buy non-biological washing powder (non-bio is only available in the UK because people here think it causes eczema, it doesn't).
What to do
1) Half fill the glasses with water and then mix in 1 teaspoon of biological washing powder/liquid into the first glass. If you have it add 1 teaspoon of the non-bio powder/liquid into the second glass. And leave the third glass with just water in it.
2) Chop the egg white into small pieces.
3) Place each piece in one of the glasses.
4) Leave the glasses in a warm place (like on a radiator or airing cupboard) overnight.
5) Inspect the bits of egg the next morning.
 |
| The bits of egg after sitting in the glasses overnight. |
What's going on?
One of the enzymes in the washing powder is known as a protease. It's a protein molecule that cuts up other proteins, some of which cause stains. But in this case the egg white, which is mainly protein, gets slowly digested by the protease. The result is a smaller bit of egg white.
I'm a big fan of proteins, they're fabulously intricate, beautiful structures that have evolved the perfect shapes and features required to do their jobs. And so I thought I'd share some of that wonder with you. This is what the protease in your washing powder looks like and (in part) you have it to thank for those nice clean pants.
Monday, 26 November 2012
Number 22: Burning steel
What do you need to make stuff burn? Just three things, oxygen, a energy source to get it all started and some fuel. These three things are there every time you light a candle or start the engine of a car. The candle wax (or petrol) is the fuel, the match (or a spark) gets is all started and the whole thing is kept going with the oxygen in the air.

So what else can we get to burn? How about steel?
Steel is mostly iron mixed with some carbon and sometimes, other metals (depending on what the steel will be used for) and its not something you'd normally think burns.
You'll need
Almost instantly you'll see part of the wool glowing red hot, very quickly this spreads through the whole clump of wool, consuming it all. Like this:

So what else can we get to burn? How about steel?
Steel is mostly iron mixed with some carbon and sometimes, other metals (depending on what the steel will be used for) and its not something you'd normally think burns.
You'll need
- Steelwool
- 9 Volt Battery
- Some old tiles or something similar that you don't mind getting scorched.
Safety
This reaction generates a lot of heat (chemists call it an exothermic reaction) and can throw out sparks so make sure there is nothing near by that might catch fire. Have a bucket of water or fire extinguisher handy. You can also end up with some small particles of steel wool being chucked up and you don't want to get them in your eyes, so wear safety goggles. Make sure there is a responsible adult supervising. Finally, because you get some smoke and sparks produced you should do this outside, you don't want to set fire to the kitchen.
What to do:
1. Fluff up the steel wool a bit. This is to make sure there is plenty of air in amongst it all.
2. Put the wool on the tiles (or whatever it it you are using)
2. Put the wool on the tiles (or whatever it it you are using)
3. Touch the terminals of the battery to the wool.
Almost instantly you'll see part of the wool glowing red hot, very quickly this spreads through the whole clump of wool, consuming it all. Like this:
What's going on:
When you burn things with carbon in them (these are known as organic compounds), like candle wax or fuel in the car, you are reacting the carbon with oxygen to make carbon dioxide gas (which has the chemical formula CO2 meaning 1 carbon and 2 oxygens). But in this case there isn't any carbon to burn nor are we lighting anything with a flame. Instead the electricity from the battery runs through the steel wool and heats it up. This happens because the electrons and ions that form the electricity collide with other particles that make up the steel wool making them move around, and heat is just the result of particles (like atoms) moving.
The heat speeds up the reaction between the iron in the wool and the oxygen in the air. This would happen anyway, without your help, just much slower (that's why things rust). And this reaction produces heat (its exothermic) which kept the reaction going until you run our of fuel (i.e. the steel wool).
When you burn candles or wood you don't end up with much left over. That's because carbon dioxide is a gas, so it floats away. But when you burn steel wool you end up with iron oxide which is a solid, hence the black stuff that's left over. One more thing, the chemical formula the iron oxide is Fe2O3 ie. 2 iron atoms (which have the chemical symbol Fe) react with 3 oxygens.
Labels:
burning,
chemistry,
combustion,
experiment,
home,
steel,
try this at home,
wire,
wool
Tuesday, 20 November 2012
A Neutron Walked into a Bar...
Christmas is fast approaching so what will geeks be asking for this year? I suggest that any science lover (young or old) worth his/her salt defiantly needs a copy of "A Neutron Walks Into a Bar ".
This fact packed book has more authors that you can shake a stick at as it's a compilation of sciency tweets that populated the twitterverse earlier this year.
A group of scienceophiles from Dublin hit upon the idea of setting twitter the daily challenge of spouting forth its favourite science facts on a given subject. And so spring 2012 was filled with the daily onslaught of a fascinating and heady mixture of jokes, quotes and quips (all in under 140 characters). Subjects ranged from maths (A googol is 1 followed by 100 zeros. 1 followed by googol zeros is a googolplex. Google's headquarters is the googleplex) to magnetism (Pigeons navigate using magnetoception via a mineral embedded in their brains called magnetite) from the particle physics (There are 6 types of quarks, known as flavours: up, down, strange, charm, bottom and top) to poetry (Darwin spoke of evolution, And brought with it revolution, Wallace, too, found the solution, We must not forget his contribution).
The result is a wonderful book (and I'm proud to have contributed to it) from which anyone and everyone can learn a thing or 2 (thousand).
If I didn't have a copy already I'd defiantly be asking for one in my stocking.
And best of all, the royalties go to the cystic fibrosis research.
This fact packed book has more authors that you can shake a stick at as it's a compilation of sciency tweets that populated the twitterverse earlier this year.
A group of scienceophiles from Dublin hit upon the idea of setting twitter the daily challenge of spouting forth its favourite science facts on a given subject. And so spring 2012 was filled with the daily onslaught of a fascinating and heady mixture of jokes, quotes and quips (all in under 140 characters). Subjects ranged from maths (A googol is 1 followed by 100 zeros. 1 followed by googol zeros is a googolplex. Google's headquarters is the googleplex) to magnetism (Pigeons navigate using magnetoception via a mineral embedded in their brains called magnetite) from the particle physics (There are 6 types of quarks, known as flavours: up, down, strange, charm, bottom and top) to poetry (Darwin spoke of evolution, And brought with it revolution, Wallace, too, found the solution, We must not forget his contribution).
The result is a wonderful book (and I'm proud to have contributed to it) from which anyone and everyone can learn a thing or 2 (thousand).
If I didn't have a copy already I'd defiantly be asking for one in my stocking.
And best of all, the royalties go to the cystic fibrosis research.
Tuesday, 30 October 2012
Number 21: Halloween slime
Halloween is almost here and so it is time for some messy mayhem! Time to roll out the classics, cornflour slime! All in funky comic book format.
I love playing with this stuff, its sooo simple and so much fun. OK it makes a bit of a mess, but it's only flour. Plus it doesn't splash, try making a big bowl of the stuff and then hitting it.
What to know more?
The cornflour water mix creates something called a non-Newtonian liquid. Basically, when you shock the liquid it turns to a solid. Ketchup is another example, that's why hitting the bottom of the bottle to get it out doesn't help much. It turns out this is really useful effect (not the ketchup), some bullet proof vests use the same principle and someone has even suggesting filling pot holes with this cornflour mix.
Sunday, 7 October 2012
Number 20: Black Worms
Firework season (in the UK) is approaching so I thought it would be fun to find a simple homemade pyrotechnic. And here it is, 'black worms' made with stuff you're bound to have around the house.
You'll need:
4. Light the gel and watch the snakes grow!
You'll need:
- Icing or powdered sugar (if you haven't got any already then just wizz some granulated sugar in a food processor).
- Bicarbonate of soda
- A pot of sand
- An alcohol based handwash gel
- A pot and a teaspoon
- Matches or a lighter
Safety:
We're using flames and flammable materials so this is best done outside and with adult supervision.
What to do:
1. Mix 2 teaspoons of icing sugar with 1/2 teaspoon of bicarbonate of soda.
4. Light the gel and watch the snakes grow!
What's going on?
The handwash gel contains ethanol which burns pretty well. This heats up the bicarbonate of soda, which gets converted to carbon dioxide, sodium carbonate and water. Meanwhile some of the sugar starts to burn i.e. it reacts with the oxygen in the air, and ends up as more carbon dioxide and water plus a whole load of stuff that comes form sugar that didn't burn completely. This is what causes the carmel smell and black sooty stuff. Then all that carbon dioxide forms bubbles in the caramelised sugar and sodium carbonate which causes it to rise up as those little worm like towers.
Sunday, 23 September 2012
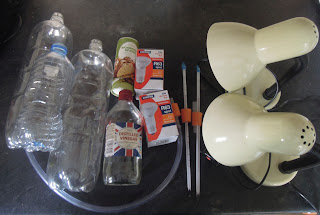
Number 19: The greenhouse effect in a bottle
The news is full of stories about climate change and the greenhouse effect. We're told that the carbon dioxide building up in the atmosphere is resulting in the planet warming. Despite all the overwhelming evidence that our planet is heating up (just look at the amount of Arctic sea ice that melted this summer) some people are still skeptical. And so here is a demo to prove to yourself and anyone else that wants to watch that carbon dioxide is most definitely a greenhouse gas.
You'll need:
 1. Drill a hole through the middle of each cork. Then carefully push the thermometers through two of them. They need to be a tight fit. Put one of the thermometers, mounted in a cork, into one of the 2 large plastic bottles.
1. Drill a hole through the middle of each cork. Then carefully push the thermometers through two of them. They need to be a tight fit. Put one of the thermometers, mounted in a cork, into one of the 2 large plastic bottles.
2. Connect up the length of tubing to the third cork. If this is proving difficult then push the empty sheath of a ball point pen through the hole and then connect the tubing to it.

3. Put the other end of the tube into the second large bottle.
4. Now take the small bottle and spoon in 3-4 teaspoons worth of bicarbonate of soda.
5. For this step you need to be quick. Pour 200ml of vinegar on top of the bicarb, then quickly push the cork thats attached to the tubing onto the bottle. Gently swirl the vinegar/bicarb mix until it stops bubbling.
TIP: IF you can't get the cork in quickly enough, then try wrapping the bicarb up in some toilet roll. Then push the bundle into the bottle, before pouring on the vinegar.
The bicarb and vinegar react to form carbon dioxide gas. This gets pushed up and out of the tubing into the second bottle. Carbon dioxide is denser than air so it then settles in the bottom of the large bottle.
TIP: You need to make sure that appart from their contents the bottles are identical. Have them the same distance from the lamps and make sure the thermometers are the same depth in the bottles.
What's going on?
The gases in the bottle are transparent, that much is obvious, after all we can see through them, and we can't tell the difference between the carbon dioxide and air bottles just by looking at them. But the gasses in the bottles are only transparent to visible light. If you could see in infrared then you'd notice that the carbon dioxide bottle blocked out more of this light than the bottle containing just air. Heat can be transferred via infrared light. So as the carbon dioxide absorbs the infrared light it heats up. And the same thing is happening to our planet.
You'll need:
- 2 x 2 litre plastic bottles (these need to be identical) and 1 smaller plastic bottle.
- 2 thermometers.
- 3 corks or rubber bungs, that fit snuggly into the necks of the bottles.
- 2 lamps fitted with 100 Watt light bulbs.
- Some vingar
- Bicarbonate of soda
- A length of tubing.
- A drill
What to do:
 1. Drill a hole through the middle of each cork. Then carefully push the thermometers through two of them. They need to be a tight fit. Put one of the thermometers, mounted in a cork, into one of the 2 large plastic bottles.
1. Drill a hole through the middle of each cork. Then carefully push the thermometers through two of them. They need to be a tight fit. Put one of the thermometers, mounted in a cork, into one of the 2 large plastic bottles.2. Connect up the length of tubing to the third cork. If this is proving difficult then push the empty sheath of a ball point pen through the hole and then connect the tubing to it.

3. Put the other end of the tube into the second large bottle.
4. Now take the small bottle and spoon in 3-4 teaspoons worth of bicarbonate of soda.
5. For this step you need to be quick. Pour 200ml of vinegar on top of the bicarb, then quickly push the cork thats attached to the tubing onto the bottle. Gently swirl the vinegar/bicarb mix until it stops bubbling.
TIP: IF you can't get the cork in quickly enough, then try wrapping the bicarb up in some toilet roll. Then push the bundle into the bottle, before pouring on the vinegar.
The bicarb and vinegar react to form carbon dioxide gas. This gets pushed up and out of the tubing into the second bottle. Carbon dioxide is denser than air so it then settles in the bottom of the large bottle.
6. Now cork the large bottle with the other thermometer.
7. Take the two lamps and put them equal distance from a bottle.
8. Turn on the lamps and watch the temperature of the bottles rise.
9. The bottle containing carbon dioxide should get hotter quicker. I saw a 5 degree centigrade difference after about 10 minutes.
TIP: You need to make sure that appart from their contents the bottles are identical. Have them the same distance from the lamps and make sure the thermometers are the same depth in the bottles.
What's going on?
The gases in the bottle are transparent, that much is obvious, after all we can see through them, and we can't tell the difference between the carbon dioxide and air bottles just by looking at them. But the gasses in the bottles are only transparent to visible light. If you could see in infrared then you'd notice that the carbon dioxide bottle blocked out more of this light than the bottle containing just air. Heat can be transferred via infrared light. So as the carbon dioxide absorbs the infrared light it heats up. And the same thing is happening to our planet.
Friday, 10 August 2012
Number 18: The bottle rocket
Rocket science isn't exactly brain surgery. A few odds and ends and a bicycle pump is all you need to build a pretty impressive rocket.
Safety first:
The rocket can take off with quite a bit of speed.
You'll need:
8. Attach the pump to the valve.
9. Stick the skewers into the ground and slide the straws onto them so that the rocket is resting on the skewers.
What's going on?
The physics involved here is really quite simple. The same principles apply as with the film canister rocket. In that case we had a container that filled up with gas as a result of a chemical reaction. With the bottle rocket the pressure build up is caused by you pumping air in. Eventually the pressure gets too great and the cork pops out. The air pressure then forces the water out, the action of the water moving down and out of the bottle in turn propels the bottle upwards.
It's another great example of Newton's 3rd law of motion which states: "To every action there is an equal and opposite reaction". So in this case the action is the water being forced out of the bottle, and the reaction is the rocket shooting off.
Safety first:
The rocket can take off with quite a bit of speed.
- Make sure there is a responsible adult present.
- Don't point the rocket towards any people or animals.
- When you launch the rocket make sure it's pointing away from you.
- Launch the rocket where there is plenty of space for it to come down. A playing field is ideal.
You'll need:
- An old bicycle inner tube.
- A cordless drill.
- An empty soda bottle.
- A cork or bung that fits snuggly into the bottle.
- Sticky tape.
- Drinking straws.
- 4 wooden skewers.
- A bit of thin card.
- A pump (a foot pump or track pump will do nicely).
- Scissors
How to build it:
1. Cut the valve out of the inner tube. Trim off the excess tubing from around the valve.
3. Push the valve through the hole in the cork. It needs to be a tight fit, so you might have to tap it in with a mallet.
4. Cut the drinking straw into 4 bits. Fold over one end of each bit of straw. Tape them to the bottle with the open end pointing towards the neck of the bottle.
5. Make a nose cone from the card and tape it to the bottom of the bottle.
6. Pour water into the bottle until it's about 1/4 full.
7. Push the cork into the bottle.
8. Attach the pump to the valve.
9. Stick the skewers into the ground and slide the straws onto them so that the rocket is resting on the skewers.
10. Make sure the rocket is pointing away from you then start the countdown and get pumping!
 |
| These are 3 successive frames from a movie shot at 30 frames per second. So you can see the rocket launches pretty quickly! |
The physics involved here is really quite simple. The same principles apply as with the film canister rocket. In that case we had a container that filled up with gas as a result of a chemical reaction. With the bottle rocket the pressure build up is caused by you pumping air in. Eventually the pressure gets too great and the cork pops out. The air pressure then forces the water out, the action of the water moving down and out of the bottle in turn propels the bottle upwards.
It's another great example of Newton's 3rd law of motion which states: "To every action there is an equal and opposite reaction". So in this case the action is the water being forced out of the bottle, and the reaction is the rocket shooting off.
Friday, 3 August 2012
Number 17: The Stopped Clock Illusion
Our brains are really astonishing things. There's a whole lot of stuff that we think we see, but really our brains are just making stuff up to fill in the gaps in our perception. But sometimes we can catch out brains out and see through the illusions they create for us. One example is with the blind spot on the backs of our eyes that I described it a while ago. Here's another example called 'the stopped clock illusion'. Maybe you've noticed before when you first look at a clock.
 You'll need:
You'll need:
A clock with a second hand.
What to do:
Its really simple. Just look at the second hand on the clock. Look away, then quickly look back.
The hand appears to stop for longer than a second. Then it ticks on around the clock face! Weird isn't it?
What's going on?
The technical name for this illusion is chronostatis (from the Greek chronos meaning 'time' and stasis meaning 'standing').
Scientist aren't completely sure what causes chronostatis. One theory is that our brains can't process information from our eyes whilst they are moving rapidly. So our brain 'backfills time' with whatever the eyes see after they've stopped moving.
If you want to know more here's a (reasonably) easy article to follow.
 You'll need:
You'll need:A clock with a second hand.
What to do:
Its really simple. Just look at the second hand on the clock. Look away, then quickly look back.
The hand appears to stop for longer than a second. Then it ticks on around the clock face! Weird isn't it?
What's going on?
The technical name for this illusion is chronostatis (from the Greek chronos meaning 'time' and stasis meaning 'standing').
Scientist aren't completely sure what causes chronostatis. One theory is that our brains can't process information from our eyes whilst they are moving rapidly. So our brain 'backfills time' with whatever the eyes see after they've stopped moving.
If you want to know more here's a (reasonably) easy article to follow.
Thursday, 19 July 2012
Number 16: Cinder toffee
No, I haven't turn this into a cookery blog. There's actually loads of cool science involved in cooking and cinder toffee is a particularly interesting example, plus as an added bonus it tastes great.
You'll need:
You'll need:
- Sugar
- Golden syrup
- A jam/jelly thermometer
- Bicarbonate of soda
- Grease proof paper
- A baking tray
- A saucepan
Safety:
The toffee mix gets very hot, be careful when handling in and make sure there's an adult helping.
What to do:
The toffee mix gets very hot, be careful when handling in and make sure there's an adult helping.
What to do:
1. Weigh out 100grams (3.5 oz) of sugar into the saucepan.
2. Add 3 tablespoons of syrup
3. Heat the mixture on a stove whilst stirring it.
4. Check the temperature of the mixture.
5. Carry on heating until it reaches 145-150oC (293-302).
5. Carry on heating until it reaches 145-150oC (293-302).
6. Quickly stir in 1 teaspoon of bicarb. It will suddenly bubble up.
7. Now pour it into the baking tray, lined with grease proof paper.
8. Leave it to cool.
9. Break it all up (best done with a hammer) and enjoy!
What's going on?
So that's a nice simple recipe for a tasty treat but where is the science?
First off there's the sugar and syrup. There are actually loads of different types of sugars, the stuff you put in your coffee and the granulated sugar used here is sucrose. It looks like this:
Hope you enjoy the toffee and whilst you do you can find out more about the science of cinder toffer here.
What's going on?
So that's a nice simple recipe for a tasty treat but where is the science?
First off there's the sugar and syrup. There are actually loads of different types of sugars, the stuff you put in your coffee and the granulated sugar used here is sucrose. It looks like this:
 |
| Sucrose |
Golden syrup is a mixture of water, sucrose and two other sugars called fructose and glucose. They look like this:
 |
| Fructose |
 |
| Glucose |
Sucrose is actually made up of a fructose and glucose molecule that have been joined together.
So why do we need these three sugars to make the toffee? Well, when they are mixed all together they interfere with crystal formation. To explain how this works let's represent each of the sugars with a different shape.
If we have one type of sugar then the molecules can pack together nice and neatly, like in the diagram. And that is exactly what happens in a crystal. But if you mix them all together they can't form ordered patterns and so you don't get crystals forming.
So if we tried to make the toffee with just one type of sugar then we'd end up with crystals forming which make for hard dense toffee (more like a boiled sweet). But by using 3 different sugars the crystals don't form and instead you end up with a brittle, crunchy, glass like toffee.
Then there's the bicarbonate of soda. You normally put this in cakes to make them rise. That's because when you heat up the bicarb it turns to carbon dioxide gas (hence the bubbles in your cakes). The same thing happens here (and its also almost the same reaction as in the film canister rockets). When you spoon the bicarb into the hot sugar it almost instantly gets converted to carbon dioxide and causes the mixture to foam up.
Hope you enjoy the toffee and whilst you do you can find out more about the science of cinder toffer here.
Thursday, 5 July 2012
Number 15: The 'impossible' balancing forks
You'll need:
- 2 identical forks
- Cocktail sticks or toothpicks
- A glass
- Matches
Safety:
Matches are involved so get adult supervisions.
What to do:
1. Lock the tines of the forks together.
2. Jam a cocktail stick through between the tines of the fork so that is sticks about 1 cm out the back.
3. Pick up the forks and place the cocktail stick over the rim of the glass with the handles pointing in towards the glass. Find the spot where the forks balance.
This already looks pretty amazing. But it gets better!
4. Now light the end of the toothpick thats pointing towards the inside of the glass.
So what's going on?
It just looks all wrong doesn't it? But the explanation is really quite simple. Half the weight of the forks is in front of the rim of the glass and the other half is behind the rim of the glass. So everything balances nicely. Sometimes physics just doesn't look right.
Subscribe to:
Comments (Atom)